Abstract

Introduction: Luspatercept inhibits select ligands of the TGF-β superfamily implicated in thalassemic erythropoiesis and promotes late-stage erythroid maturation (Suragani RN, et al. Nat Med 2014;20:408-414). This leads to greater red blood cell (RBC) output from thalassemic marrow and reduces transfusion dependence in TDT (Cappellini MD, et al. N Engl J Med 2020;382:1219-1231). The underlying mechanisms for this clinical outcome are not well understood in a syndrome involving significant iron overload and cyclical stimulation of erythropoiesis between transfusions. Here we report novel physiological and clinical insights from a BELIEVE trial (NCT02604433) biomarker analysis, which demonstrate that hepcidin and erythropoietic changes in TDT lead to iron redistribution from macrophages to hepatocytes on luspatercept.
Methods: 336 TDT patients ≥ 18 years of age who took part in the BELIEVE study, a multicenter, randomized, double-blind, placebo-controlled phase 3 trial (Cappellini et al. 2020), were randomized 2:1 to receive luspatercept or placebo subcutaneously every 21 days for 48 weeks. This ethically approved study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Transfusion iron loading rate (ILR) was calculated assuming 1 unit = 200 mg Fe. Liver iron content (LIC) was measured by R2 MRI and R2* MRI, and total body iron stores by Angelucci formula. Serum was assayed for ferritin (SF) nephelometrically (Covance lab), and hepcidin, erythroferrone (ERFE), growth differentiation factor (GDF)15, and transferrin receptor (sTfR1) by ELISA at Intertek (San Diego, CA). Median and interquartile ranges are shown; P value < 0.05 was deemed significant.
Results: Within 48 weeks on luspatercept, transfusion iron loading fell by 1.6 g (8 RBC units, ILR difference −0.08 mg Fe/kg/d ± 0.07, range −0.4 to 0.2). Regardless of thalassemic genotype, SF fell by 269.3 ± 963.7 and earliest at 12 weeks by 103.6 ± 690.3 µg/L (both P < 0.0001), with no change on placebo, indicating reduced macrophage iron. However, despite unchanged chelation exposure, no reduction in LIC (5.7 to 6.7 mg/g dw) or calculated body iron stores (3.6 to 4.2 g, not significant) occurred on luspatercept, even though saved iron loading from transfusion was 44% (1.6 g/3.6 g) of baseline body iron stores. On luspatercept, but not placebo, hepcidin fell by 53%, while erythropoietin (EPO), ERFE, GDF15, sTfR1, and reticulocytes rose by 93%, 51%, 59%, 66%, and 112%, respectively (all P < 0.0001).
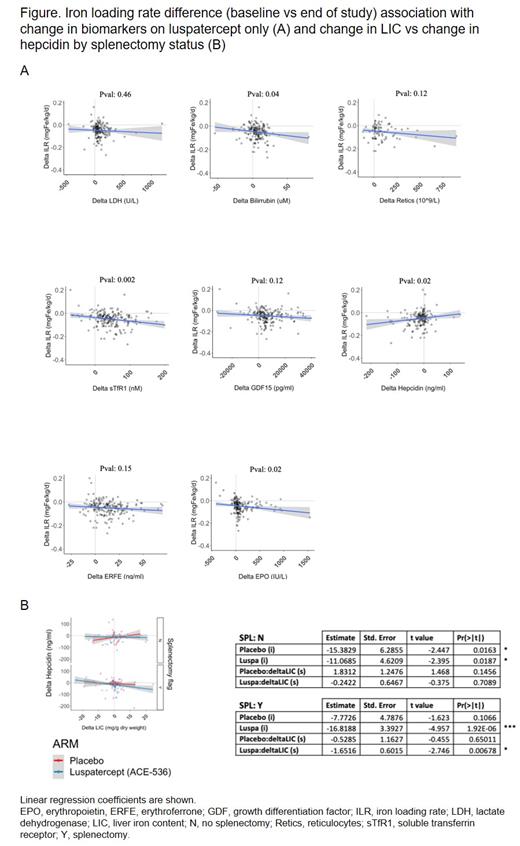
71% (120/169) patients with and 47% without (17/36) ILR reduction had negative SF trends (P < 0.0001). In patients with SF reduction on luspatercept, bilirubin and LDH rose 50% and 67% (P < 0.0001) indicating increased RBC hemolysis from residual (effective and ineffective) erythropoiesis. ILR change correlated with changes in EPO, hepcidin, sTfR1, and bilirubin, but not with changes in ERFE, GDF15, reticulocytes, or LDH (Figure A). Hepcidin reduction was related to LIC increase post splenectomy (Figure B), suggesting a role for the spleen in preventing hemolytic iron redistribution to the liver. Decrease in SF thus associates with erythroid iron incorporation (sTfR1), hence RBC production that reduces transfusion needs (falling ILR) but enhances hemolytic rerouting of iron to the liver.
In a mixed-effect linear regression analysis, transfusion, LIC, baseline SF, time, and treatment predicted SF changes in a benchmark model. We found high baseline LIC lessens the SF outcome, and hepcidin, EPO, and bilirubin jointly explained 66% of the treatment effect of luspatercept on SF.
Conclusions: Luspatercept-increased ERFE, likely as a result of increased production or higher frequency of late-stage erythroblasts, partially reduces hepcidin production. Lower hepcidin mobilizes iron stores to facilitate bulk hemoglobinization, erythropoietic response, and transfusion burden reduction. Luspatercept leads to SF reduction that marks hepcidin-dependent iron egress from macrophage compartment to plasma. This relocated iron, variably utilized by thalassemic erythron, refluxes back to plasma for hepatocyte and extrahepatic iron uptake, or as heme iron shunts into the liver through hemolytic pathways from intramarrow ineffective erythropoiesis or peripheral breakdown of newly made thalassemic RBC. Macrophage to hepatocyte iron redistribution in TDT appears to be a mechanism of luspatercept.
Garbowski: Imara: Consultancy; Vifor: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy. Ugidos: Bristol Myers Squibb: Current Employment. Risueño: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Suragani: Acceleron Pharma: Current Employment, Current equity holder in publicly-traded company. Shetty: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Vodala: BMS: Current Employment, Other: stock options. Thakurta: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Schwickart: BMS (Celgene): Current equity holder in publicly-traded company, Ended employment in the past 24 months, Other, Patents & Royalties; Exelixis: Current Employment, Current equity holder in publicly-traded company. Porter: Celgene (BMS): Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Vifor: Honoraria, Membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria; Protagonism: Honoraria; La Jolla Pharmaceuticals: Honoraria; bluebird bio, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal